
Hidefumi Iwashita, Nobuaki Shiraki, Daisuke Sakano, Takashi Ikegami, Masanobu Shiga, Kazuhiko Kume, Shoen Kume* (* corresponding author).
Secreted Cerberus1 as a marker for quantification of definitive endoderm differentiation of the pluripotent stem cells
PLoS ONE 8(5): e64291, 2013.
To date, CXCR4 and E-cadherin double-positive cells detected by flow cytometry have been used to identify the differentiation of embryonic stem (ES) cells or induced pluripotent stem (iPS) cells into definitive endoderm (DE) lineages. Quantification of DE differentiation from ES/iPS cells by using flow cytometry is a multi-step procedure including dissociation of the cells, antibody reaction, and flow cytometry analysis. To establish a quick assay method for quantification of ES/iPS cell differentiation into the DE without dissociating the cells, we examined whether secreted Cerberus1 (Cer1) protein could be used as a marker. Cer1 is a secreted protein expressed first in the anterior visceral endoderm and then in the DE. The amount of Cer1 secreted correlated with the proportion of CXCR4+/E-Cadherin+ cells that differentiated from mouse ES cells. In addition, we found that human iPS cell-derived DE also expressed the secreted CER1 and that the expression level correlated with the proportion of SOX17+/FOXA2+ cells present. Taken together, these results show that Cer1 (or CER1) serves as a good marker for quantification of DE differentiation of mouse and human ES/iPS cells.
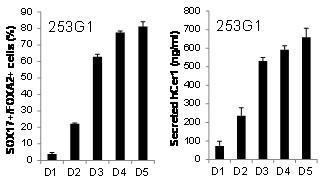
Fig. The supernatant was sampled 24 h after replacement with fresh media on differentiation days 1 to 5 (D1 to D5) of the human iPS cell line (253G1) .