
Sasai N, Saitoh N, Saitoh H, Nakao M (2013) The Transcriptional Cofactor MCAF1/ATF7IP Is Involved in Histone Gene Expression and Cellular Senescence.
PLoS ONE 8(7): e68478. doi:10.1371/journal.pone.0068478
Cellular senescence is post-mitotic or oncogene-induced events combined with nuclear remodeling. MCAF1 (also known as hAM or ATF7IP), a transcriptional cofactor that is overexpressed in various cancers, functions in gene activation or repression, depending on interacting partners. In this study, we found that MCAF1 localizes to PML nuclear bodies in human fibroblasts and non-cancerous cells. Interestingly, depletion of MCAF1 in fibroblasts induced premature senescence that was characterized by cell cycle arrest, SA-β-gal activity, and senescence-associated heterochromatic foci (SAHF) formation. Under this condition, core histones and the linker histone H1 significantly decreased at both mRNA and protein levels, resulting in reduced nucleosome formation. Consistently, in activated Ras-induced senescent fibroblasts, the accumulation of MCAF1 in PML bodies was enhanced via the binding of this protein to SUMO molecules, suggesting that sequestration of MCAF1 to PML bodies promotes cellular senescence. Collectively, these results reveal that MCAF1 is an essential regulator of cellular senescence.
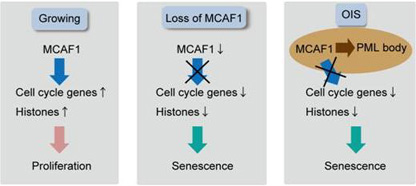
Figure. In growing cells, MCAF1 maintains cell proliferation by activating transcription of cell cycle and histone genes (left). MCAF1 knockdown downregulates cell cycle and histone genes, and results in premature senescence (middle). In oncogene-induced senescent (OIS) cells, MCAF1 is recruited to PML bodies through binding to SUMO2/3. This may inhibit MCAF1 function to maintain expression of cell cycle genes and histones (right).