
Atsuhiro Taguchi, Yusuke Kaku, Tomoko Ohmori, Sazia Sharmin, Minetaro Ogawa, Hiroshi Sasaki, and Ryuichi Nishinakamura
Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells (2013) Cell Stem Cell Dec 12. Epub ahead of print
Recapitulating three-dimensional (3D) structures of complex organs, such as the kidney, from pluripotent stem cells (PSCs) is a major challenge. Here, we define the developmental origins of the metanephric mesenchyme (MM), which generates most kidney components. Unexpectedly, we find that posteriorly located T+ MM precursors are developmentally distinct from Osr1+ ureteric bud progenitors during the postgastrulation stage (Figs. 1 & 2), and identify phasic Wnt stimulation and stage-specific growth factor addition as molecular cues that promote their development into the MM (Fig. 3). We then use this information to derive MM from PSCs. These progenitors reconstitute the 3D structures of the kidney in vitro, including glomeruli with podocytes and renal tubules with proximal and distal regions and clear lumina (Fig. 4). Furthermore, the glomeruli are efficiently vascularized upon transplantation. Thus, by reevaluating the developmental origins of metanephric progenitors, we have provided key insights into kidney specification in vivo and taken important steps toward kidney organogenesis in vitro.
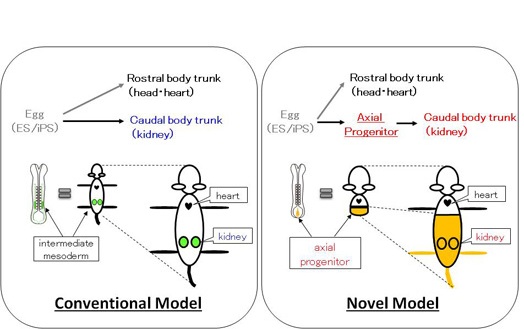
Figure 1: The lower half of the body is derived from axial stem cells
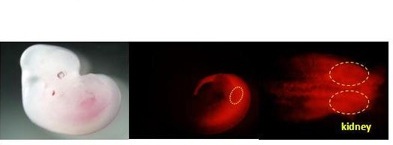
Figure 2: The kidney is originated from the axial stem-like cells
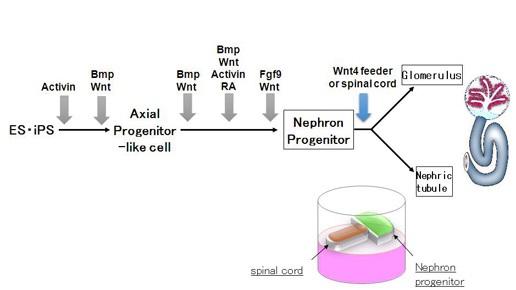
Figure 3: The strategy to generate the kidney in vitro
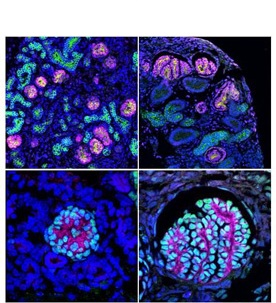
Figure 4: The kidney structures generated from pluripotent stem cells in vitro
(upper left) the kidney structures generated from mouse ES cells
(upper right) the kidney structures generated from human iPS cells
(lower left) The glomerulus generated from mouse ES cells
(lower right) The glomerulus generated from human iPS cells