
Kentaro Noi, Daisuke Yamamoto, Shingo Nishikori, Ken-ichi Arita-Morioka, Takayuki Kato, Toshio Ando, and Teru Ogura. High-Speed Atomic Force Microscopic Observation of ATP-Dependent Rotation of AAA+ Chaperone p97. Structure, in press
p97 (also called VCP and CDC-48) is an AAA+ chaperone, which consists of a substrate/cofactorbinding N domain and two ATPase domains (D1 and D2), and forms a homo-hexameric ring. p97 plays crucial roles in a variety of cellular processes such as the ubiquitin-proteasome pathway, the endoplasmic reticulum-associated protein degradation, autophagy, and modulation of protein aggregates. Mutations in human p97 homolog VCP are linked to neurodegenerative diseases. The key mechanism of p97 in these various functions has been proposed to be the disassembly of protein complexes. To understand the molecular mechanism of p97, we studied the conformational changes of hexameric CDC-48.1, a Caenorhabditis elegansp97 homolog, using high-speed atomic force microscopy. In the presence of ATP, the N-D1 ring repeatedly rotates ~23 ± 8 ° clockwise and resets relative to the D2 ring. Mutational analysis reveals that this rotation is induced by ATP binding to the D2 domain.
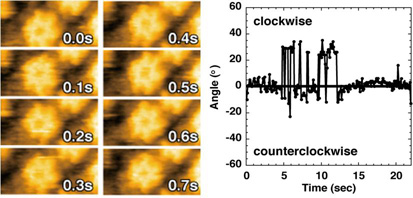
Figure. ATP-Dependent Rotational Movements of p97. (left) Successive AFM images of p97 taken in the presence of ATP. (right) Time course of angular variation of diametric lines. Clockwise rotation is indicated by plus degrees, and counterclockwise one is indicated by minus degrees.
