

Organogenesis -Molecular mechanisms of kidney development
Our research interest is to elucidate molecular mechanisms in organogenesis, especially kidney development. We also aim at derivation of kidney progenitors from stem cells, by utilizing knowledge obtained from molecular genetics.
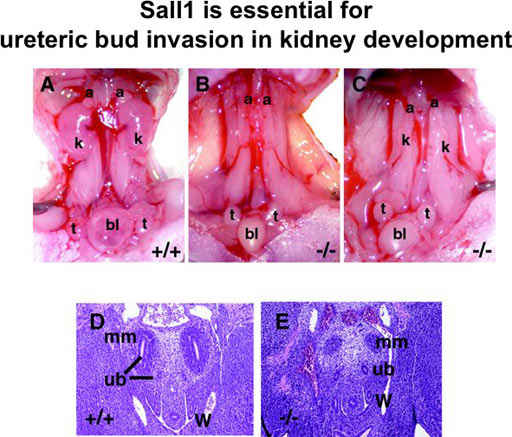
Sall1 is essential for the kidney development (Fig. 1)
The kidney develops in three stages: pronephros, mesonephros, and metanephros. Many of the genes expressed in the metanephros are also found in the pronephros. Animal caps, a presumptive ectoderm of Xenopus embryos at the blastula stage, differentiate into three-dimensional pronephric tubules in three days in chemically defined saline solution upon treatment with activin and retinoic acid. We have used this system to identify molecules expressed in pronephros and potentially in mesonephros and metanephros. One of the genes we isolated was Xsal-3, a newly identified sal member of Xenopus, which was expressed in the pronephros and the brain. We then cloned a member of the murine sal family from the developing kidney, which proved to be a mouse homolog of human SALL1.
SALL1 is a mammalian homolog of the Drosophila region-specific homeotic gene spalt (sal) and heterozygous mutations in SALL1 in humans lead to Townes-Brocks syndrome. We isolated a mouse homolog of SALL1 (Sall1) and found that mice deficient in Sall1 die in the perinatal period and that kidney agenesis or severe dysgenesis are present. Sall1 is expressed in the metanephric mesenchyme surrounding ureteric bud and homozygous deletion of Sall1 results in an incomplete ureteric bud outgrowth, a failure of tubule formation in the mesenchyme and an apoptosis of the mesenchyme. This phenotype is likely to be primarily caused by the absence of the inductive signal from the ureter, as the Sall1 deficient mesenchyme is competent regarding epithelial differentiation. Therefore Sall1 is essential for ureteric bud invasion, the initial key step for metanephros development.
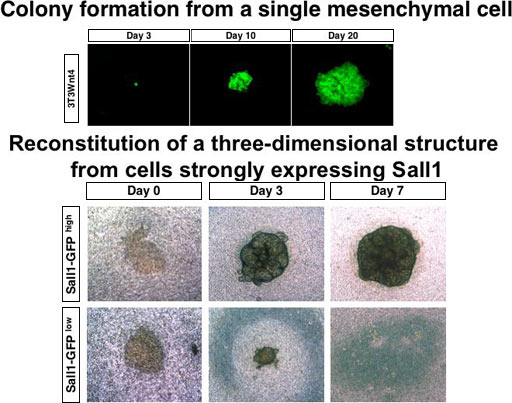
Identification of multipotent progenitors in the embryonic mouse kidney by a novel colony-forming assay (Fig. 2)
Renal stem or progenitor cells with a multilineage differentiation potential remain to be isolated, and the differentiation mechanism of these cell types in kidney development or regeneration processes has been unknown. In an attempt to overcome this issue, we set up an in vitro culture system using NIH3T3 cells stably expressing Wnt4 (3T3Wnt4) as a feeder layer, in which a single renal progenitor in the metanephric mesenchyme forms colonies consisting of several types of epithelial cells that exist in glomeruli and renal tubules. We found that only cells strongly expressing Sall1 (Sall1-GFP high cells), a zinc finger nuclear factor essential for kidney development, form colonies, and that they reconstitute a three-dimensional kidney structure in an organ culture setting. We also found that Rac- and c-Jun N-terminal kinase (JNK)-dependent planar cell polarity (PCP) pathways downstream of Wnt4 positively regulate the colony size, and that the JNK pathway is also involved in mesenchymal-to-epithelial transformation of colony-forming progenitors. Thus our colony-forming assay, which identifies multipotent progenitors in the embryonic mouse kidney, can be used for examining mechanisms of renal progenitor differentiation.
Sall4 is essential for embryonic stem cell proliferation, and cooperates with Sall1 in anorectal, heart, brain, and kidney development (Fig. 3)
Mutations in SALL4, the human homolog of the Drosophila homeotic gene spalt (sal), cause the autosomal dominant disorder known as Okihiro syndrome. We show that a targeted null mutation in Sall4 lead to lethality during peri-implantation. Growth of the inner cell mass from the knockout blastocysts was reduced, and Sall4-null embryonic stem cells proliferated poorly with no aberrant differentiation. Furthermore, we demonstrated that anorectal and heart anomalies in Okihiro syndrome are caused by Sall4 haploinsufficiency and that Sall4/Sall1 heterozygotes exhibited an increased incidence of anorectal and heart anomalies, exencephaly, and kidney agenesis. Sall4 and Sall1 formed heterodimers, and a truncated Sall1 caused mislocalization of Sall4 in the heterochromatin; thus, some symptoms of Townes-Brocks syndrome caused by SALL1 truncations could result from SALL4 inhibition.
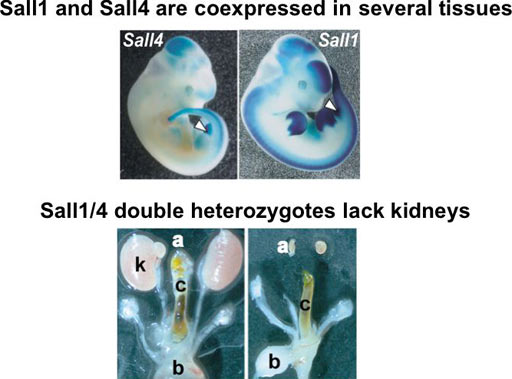
Fig. 3
Kif26b, a kinesin family gene, regulates adhesion of the embryonic kidney mesenchyme
The kidney develops through reciprocal interactions between two precursor tissues: the metanephric mesenchyme and the ureteric bud. We previously demonstrated that the zinc finger protein Sall1 is essential for ureteric bud attraction toward the mesenchyme. Here we show that Kif26b , a kinesin family gene, is a downstream target of Sall1 and that disruption of this gene causes kidney agenesis because of impaired ureteric bud attraction. In the Kif26b -null metanephros, compact adhesion between mesenchymal cells adjacent to the ureteric buds and the polarized distribution of integrin α8 were impaired, resulting in failed maintenance of Gdnf , a critical ureteric bud attractant. Overexpression of Kif26b in vitro caused increased cell adhesion through interactions with non-muscle myosin. Thus Kif26b is essential for kidney development because it regulates the adhesion of mesenchymal cells in contact with ureteric buds. To the best of our knowledge, this is the first report that a kinesin deficiency can cause the lack of an entire organ. Uchiyama et al. Proc. Natl. Acad. Sci. USA 2010
The phosphatase Dullard negatively regulates BMP signalling and is essential for nephron maintenance after birth
Most kidney nephron components, including glomeruli and renal tubules, derive from the metanephric mesenchyme. The overall differentiation into each component finishes at birth, but the molecular events linking the perinatal and adult kidneys remain elusive. Dullard was cloned from Xenopus kidneys, and encodes a phosphatase that negatively regulates BMP signalling. Here we report that Dullard deletion in the murine metanephric mesenchyme leads to failure of nephron maintenance after birth, resulting in lethality before adulthood. The nephron components are lost by massive apoptosis within 3 weeks after birth, leading to formation of a large hollow with a thin-layered cortex and medulla. Phosphorylated Smad1/5/8 is upregulated in the mutant nephrons, probably through cell-autonomous inhibitory effects of Dullard on BMP signalling. Importantly, administration of the BMP receptor kinase inhibitor LDN-193189 partially rescued the defects caused by Dullard deletion. Thus, Dullard keeps BMP signalling at an appropriate level, which is required for nephron maintenance in the postnatal period.Sakaguchi et al. Nat Commun. 2013
Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells
Recapitulating three-dimensional (3D) structures of complex organs, such as the kidney, from pluripotent stem cells (PSCs) is a major challenge. Here, we define the developmental origins of the metanephric mesenchyme (MM), which generates most kidney components. Unexpectedly, we find that posteriorly located T+ MM precursors are developmentally distinct from Osr1+ureteric bud progenitors during the postgastrulation stage, and identify phasic Wnt stimulation and stage-specific growth factor addition as molecular cues that promote their development into the MM . We then use this information to derive MM from PSCs. These progenitors reconstitute the 3D structures of the kidney in vitro, including glomeruli with podocytes and renal tubules with proximal and distal regions and clear lumina . Furthermore, the glomeruli are efficiently vascularized upon transplantation. Thus, by reevaluating the developmental origins of metanephric progenitors, we have provided key insights into kidney specification in vivo and taken important steps toward kidney organogenesis in vitro. Taguchi et al. Cell Stem Cell 2014