
Yohei Sasagawa*, Mieko Otani, Nahoko Higashitani, Atsushi Higashitani, Ken Sato, Teru Ogura, and Kunitoshi Yamanaka*. (2009) Caenorhabditis elegans p97 controls germline specific sex determination by controlling TRA-1 level in a CUL-2 dependent manner. J. Cell Sci. 122: 3663-3672. (*equal contribution)
p97/CDC-48 is a ubiquitin-selective AAA (ATPases associated with diverse cellular activities) chaperone and its key function is to disassemble protein complexes. p97 functions in diverse cellular processes including endoplasmic reticulum–associated degradation, membrane fusion, and meiotic and mitotic progression. However, its cellular functions in development have not yet been clarified. Here, we present data that p97 is involved in the switching from spermatogenesis to oogenesis in the germline of the Caenorhabditis elegans hermaphrodite. We found that thecdc-48.1 deletion mutant produced less sperm than wild-type and thus showed a decreased brood size. The cdc-48.1 mutation suppressed the sperm over-producing phenotypes of fbf-1 and fem-3 ( gf ) mutants. p97/CDC-48UFD-1-NPL-4 interacted with the E3 ubiquitin ligase CUL-2 complex through NPL-4 binding to Elongin C. Furthermore, TRA-1A, which is the terminal effector of the sex determination pathway and is regulated by CUL-2–mediated proteolysis, accumulated in the cdc-48.1 mutant. Proteasome activity was also required for the brood size determination and sperm/oocyte switch. Our results demonstrate that C. elegans p97/CDC-48UFD-1-NPL-4 controls the sperm/oocyte switch by regulating the CUL-2–mediated TRA-1A proteasome degradation.
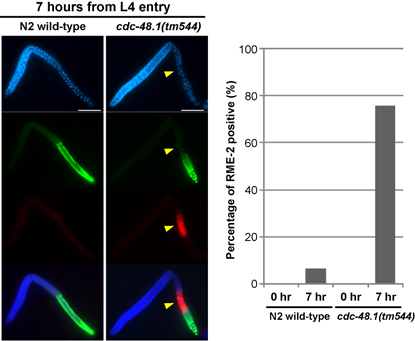
Fig. 1. The sperm-oocyte switch in cdc-48.1(tm544) hermaphrodites takes place at an earlier stage than in the wild type. Worms were first synchronized at the L3 stage, and incubated for several hours until they grew up to the L4 stage. Gonads were dissected, fixed and stained with DAPI (blue), anti-MSP antibody (green) and anti-RME-2 antibody (red) at 0 and 7 hours from L4 entry. RME-2 was used as an oogenesis marker. At 0 hour from L4 entry, no worm expressed RME-2. While only 6.3% of gonads faintly expressed RME-2 in the wild type at 7 hours from L4 entry, 75.6% of gonads expressed RME-2 in cdc-48.1(tm544) . pancreas.
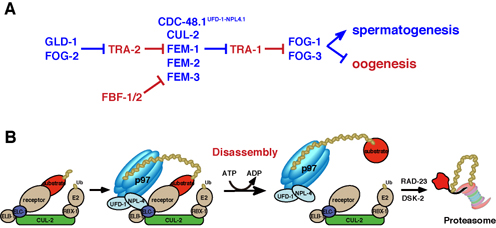
Fig. 2. Model for p97 involvement in regulation of substrate fate.
(A) CDC-48.1UFD-1–NPL-4.1 is involved in the regulation of TRA-1 degradation in combination with CUL-2 ubiquitin ligase containing FEM-1/-2/-3 as a substrate recognition receptor. TRA-1 degradation is not efficient in the cdc-48.1(tm544) mutant and thus switching from spermatogenesis to oogenesis occurs earlier than in wild-type. Proteins that promote spermatogenesis are blue, and those that promote oogenesis are red.
(B) Proposed model of p97 requirement for disassembly of the ubiquitinated substrate from the CUL-2 ubiquitin ligase complex. p97 may disassemble the substrate by changing its conformation using ATP-hydrolyzing energy.