

Our goal is to unravel molecular and cellular mechanisms underlying development of the hematopoietic and vascular systems. By using an in vitro differentiation system of murine embryonic stem cells, we are trying to identify the genetic program by which the self-renewal capacity as well as multiple potentials of the hematopoietic stem cell is established. Our system also makes it possible to elucidate cell biological functions of angiogenic growth factors and transcription factors, providing a clue to how the morphogenic activity of endothelial cells is regulated by angiogenic stimuli to form a hierarchically organized vascular architecture.
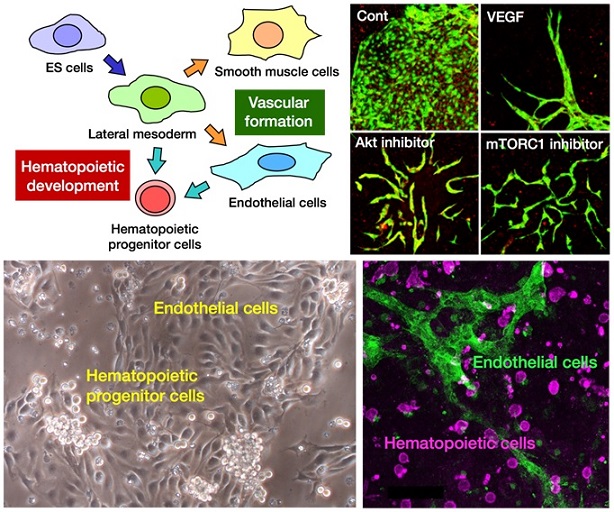
Upper left: Schematic diagram of in vitro differentiation of ES cells into the hematopoietic and vascular lineages. Upper right: Morphological changes of ES cell-derived vascular endothelial cells induced by various angiogenic stimuli. Lower left: Hematopoietic and endothelial differentiation of ES cell-derived lateral mesodermal cells in culture. Lower right: Generation of hematopoietic cells and endothelial cells from an isolated single hemogenic endothelial cell that was derived from ES cells.