
Yuka Matsushita-Ishiodori, Kunitoshi Yamanaka, and Teru Ogura (2007) The C. elegans homologue of the spastic paraplegia protein, spastin, disassembles microtubules. Biochem. Biophys. Res. Commun. 359, 157-162.
Mutations in human spastin (SPG4) cause an autosomal dominant form of hereditary spastic paraplegia. Sequence analysis revealed that spastin contains the AAA (ATPases associated with diverse cellular activities) domain in the C-terminal region. Recently, it was reported that spastin interacts dynamically with microtubules (MTs) and displays MT-severing activity. A plausible Caenorhabditis elegans homologue of spastin (SPAS-1) has been identified by homology search and phylogenetic analyses. To understand the function of the spastin homologue, we characterized the spas-1 deletion mutant and analyzed spas-1 expression regulation in C. elegans . SPAS-1 was localized with cytoskeletons at the perinuclear region. We found that MTs were intensely stained at the centrosomal region in the deletion mutant. Furthermore, overexpression of SPAS-1 caused disassembly of MT network in cultured cells, while ATPase-deficient SPAS-1 did not (Figure). These results indicate that C. elegans SPAS-1 plays an important role in MT dynamics. We also found that two kinds of products were generated from spas-1 by alternative splicing in a developmental stage-dependent manner.
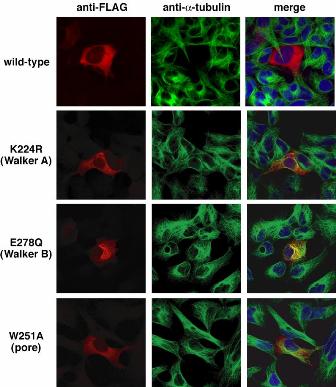
Figure. MT disassembly activity of SPAS-1. FLAG-epitope tagged C. elegans SPAS-1 and mutated SPAS-1 (K224R, E278Q and W251A) were overexpressed in HEK293 cells. Transfected cells were immunostained with anti-FLAG (left) and anti- a -tubulin (middle) antibodies. Merged images were also shown (right). Wild-type SPAS-1, but none of mutants, possessed MT disassembly activity.