
Shingo Nishikori * , Masatoshi Esaki * , Kunitoshi Yamanaka, Shinya Sugimoto, and Teru Ogura (2011) Positive cooperativity of the p97 AAA ATPase is critical for essential functions. J. Biol. Chem. , in press (*equal contribution).
p97 is composed of two conserved AAA (ATPases associated with diverse cellular activities) domains, which form a tandem hexameric ring. We characterized the ATP-hydrolysis mechanism of CDC-48.1, a p97 homolog ofCaenorhabditis elegans. The ATPase activity of the N-terminal AAA domain was very low at physiological temperature, whereas the C-terminal AAA domain showed high ATPase activity in a coordinated fashion with positive cooperativity. The cooperativity and coordination are generated by different mechanisms since a noncooperative mutant still showed the coordination. Interestingly, the growth speed of yeast cells strongly related to the positive cooperativity rather than the ATPase activity itself (Figure), suggesting that the positive cooperativity is critical for the essential functions of p97.
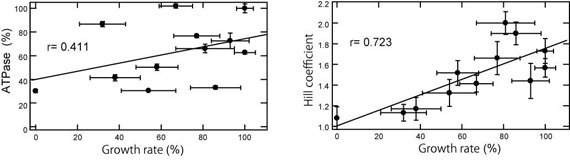
Figure: Relationships between the yeast growth rate and Hill coefficient or ATPase activity.
Growth rates of yeast cells expressing mutant p97 were determined from log-phase growth. The growth rate of mutant cells was plotted against ATPase activity (A) and Hill coefficient (B) of purified p97 mutants. Hill coefficient being greater than 1 represents positive cooperativity in the system. Correlation coefficient ( r ) was determined by fitting the data as a linear model. The closer to 1 correlation coefficient is, the higher relationships present.