
Shingo Nishikori, Kunitoshi Yamanaka, Toshihiko Sakurai, Masatoshi Esaki, and Teru Ogura. p97 homologs from C. elegans , CDC-48.1 and CDC-48.2, suppress the aggregate formation of huntingtin exon1 containing expanded polyQ repeat. Genes to Cells (2008) 13 , 827–838
Polyglutamine (polyQ)-expanded proteins are associated with cytotoxicity in some neurodegenerative disorders such as Huntington’s disease. We have reported that the aggregation of the polyQ-expanded protein is partially suppressed by co-expression of either of two homologs of an AAA chaperone p97, CDC-48.1 or CDC-48.2, in Caenorhabdits elegans , but how p97 regulates the aggregation of polyQ-expanded proteins remains unclear. Here we present direct evidence that CDC-48.1 and CDC-48.2 suppress the aggregation of a huntingtin (Htt) exon1 fragment containing an expanded polyQ repeat in vitro . CDC-48.1 and CDC-48.2 bound the Htt exon1 fragment directly, and suppressed the formation of SDS-insoluble aggregates of Htt fragments containing 53 glutamine residues (HttQ53) independently of nucleotides. CDC-48.1 and CDC-48.2 also modulated the oligomeric states of HttQ53 during the aggregate formation. In the absence of CDC-48.1 and CDC-48.2, HttQ53 formed 70-150 kDa oligomers, while 300-500 kDa oligomers as well as 70-150 kDa oligomers accumulated in the presence of CDC-48.1 and CDC-48.2. Taken together, these results suggest that p97 plays a protective role in neurodegenerative disorders by directly suppressing the protein aggregation as a molecular chaperone.
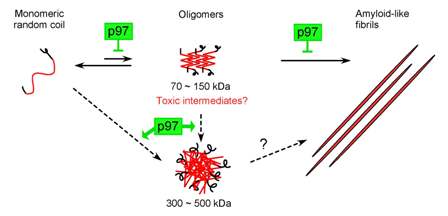
Figure: Model for modulation of polyQ aggregation by p97. CDC-48.1 and CDC-48.2 modulate the oligomer states of polyQ-expanded proteins, and suppress formation of amyloid-like fibrils.