
Revealing the Pressure Environment surrounding Fetal Brain Development
— First successful in utero measurement of intraventricular pressure —
Mami Akaike†, Jun Hatakeyama†*, Yuta Nakashima, Kenji Shimamura*.
Measuring intraventricular pressure in developing mouse embryos: Uncovering a repetitive mechanical cue for brain development.
Dev Growth Differ. 2025 May 13
†equal contribution, *corresponding author
A research group led by Associate Professor Jun Hatakeyama and Professor Kenji Shimamura from the Department of Brain Morphogenesis at the Institute of Molecular Embryology and Genetics, Kumamoto University, in collaboration with Assistant Professor Mami Akaike and Associate Professor Yuta Nakashima from the Faculty of Advanced Science and Technology, has successfully developed a technique for quantitatively and in real-time measurement of the intraventricular pressure in the fetal mouse brain, shedding new light on the role of mechanical stimuli in brain development (Fig. 1).
Measuring pressure inside the developing brain ventricles—only about 1 mm³ in volume—had long been a technical challenge. In this study, the researchers inserted a thin glass capillary directly connected to the pressure sensor into the ventricles of mouse embryos (E12.5 to E16.5) in utero, and monitored the pressure dynamics over time.
We found that the baseline intraventricular pressure increased gradually with developmental stage in vitro. However, when measured in utero, the intraventricular pressure was several times higher than that of isolated embryos, primarily due to pressure exerted by the uterus. Interestingly, this uterine-derived pressure decreased as development progressed. Moreover, for the first time, we observed that the intraventricular pressure fluctuated in ~30-second cycles, synchronized with maternal uterine contractions. This indicates that the fetal brain is subjected to rhythmic mechanical stimulation during development.
Since cerebrospinal fluid is incompressible, these pressure fluctuations likely compress the neuroepithelium along its apical–basal axis in a periodic manner. Whether such mechanical cues play a functional role in shaping the brain is an important question for future investigation.
Additionally, when the brain vesicles of mouse embryos (E10.5–E15.5) were punctured to release the internal pressure ex utero, we observed that the neuroepithelium rapidly contracted and thickened (Fig. 2). This suggests that the neuroepithelial sheet, composed of neural stem cells, was stretched horizontally by internal pressure—much like the surface of a balloon. Notably, this response was no longer observed by E15.5, suggesting developmental regulation of this mechanical sensitivity.
This study provides a novel insight into the mechanical environment during brain development and opens new avenues for understanding developmental disorders of the brain. The findings may also contribute to advancing brain organoid technology, artificial womb development, and regenerative medicine.
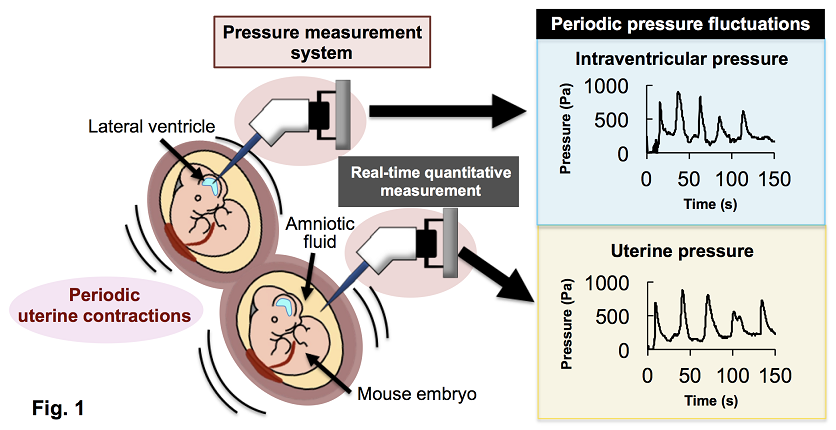
Fig. 1: Intraventricular and uterine pressure measurement
A glass capillary connected to the pressure sensors was inserted into the lateral ventricles of mouse fetuses and the surrounding amniotic fluid. Both intraventricular and uterine pressures were successfully measured and found to fluctuate rhythmically.
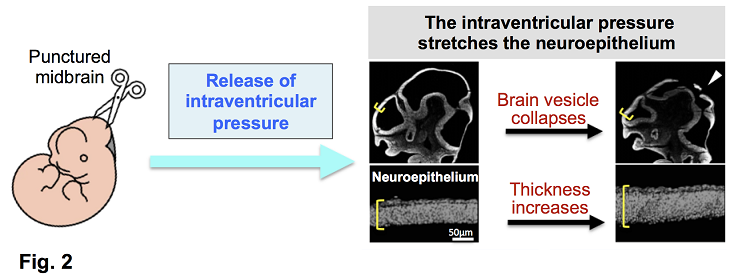
Fig. 2: Brain vesicle perforation experiment
When encephalic vesicles of embryos (E10.5–E15.5) were punctured, they collapsed immediately, and the neuroepithelial sheet became thicker. This indicates that the ventricular lumen is under positive pressure and that the neuroepithelia are normally stretched under tension.
Title: Measuring intraventricular pressure in developing mouse embryos: Uncovering a repetitive mechanical cue for brain development
Authors: Mami Akaike†, Jun Hatakeyama†*, Yuta Nakashima, Kenji Shimamura*
†Equal contribution, *Corresponding author
Journal: Development, Growth & Differentiation (Published online May 13, 2025)
DOI: https://doi.org/10.1111/dgd.70010