
Endocytosed dsRNAs induce lysosomal membrane permeabilization that allows cytosolic dsRNA translocation for Drosophila RNAi responses
Tsubasa Tanaka, Tamaki Yano, Shingo Usuki, Yoko Seo, Kento Mizuta, Maho Okaguchi, Maki Yamaguchi, Kazuko Hanyu-Nakamura, Noriko Toyama-Sorimachi, Katja Brückner, and Akira Nakamura
Nature Communications 15, 6993 (2024)
RNA interference (RNAi) is a gene silencing mechanism triggered by the entry of double-stranded RNAs (dsRNAs) into the cytoplasm. RNAi-based nucleic acid drugs have been approved as therapeutic agents. In the cytoplasm, dsRNA is processed into small interference RNA (siRNA) of approximately 21 nucleotides, which is then incorporated into the RNAi effector Ago2 to form an RNA-induced silencing complex (RISC). RISC silences genes by cleaving target RNAs that have complementary sequences to the bound siRNA.
In many animal cells, extracellular dsRNA can be internalized by endocytosis and used for RNAi reactions. Gene knockdown screens (so called soaking RNAi) have been widely used to take advantage of this property. However, it was not clear how dsRNAs in endocytosed vesicles are transferred across the endo/lysosomal membrane into the cytoplasm. Only the dsRNA transporter, called SID-1, is known to directly import extracellular dsRNA into the cytoplasm and promote systemic RNAi in the nematode, Caenorhabditis elegans. However, homologs of SID-1 do not exist in the fruit fly Drosophila, and extracellular dsRNAs would be taken up by an unknown mechanism in cultured Drosophila cells, where soaking RNAi screens have been widely used.
In this study, Assistant Professor Dr. Tsubasa Tanaka (retiring March 2023) and his colleagues in the Department of Germline Development, Institute of Molecular Embryology (IMEG), Kumamoto University showed that endocytosed dsRNAs induce lysosomal membrane permeabilization (LMP) in cultured Drosophila S2 cells, allowing dsRNAs to enter the cytosol. Furthermore, we found that such dsRNA-mediated LMP requires the lysosomal Cl–/H+ antiporter consisting of ClC-b/DmOstm1. In clc-b or dmostm1 knockout S2 cells, extracellular dsRNAs are endocytosed and normally reach the lysosomes. However, dsRNAs in vesicles were unable to enter the cytoplasm. On the other hand, pharmacological induction of LMPs with chloroquine and other substances restored the RNAi responses by extracellular dsRNAs even in the absence of clc-b or dmostm1. Furthermore, in clc-b or dmostm1 knockout flies, RNAi induction by dsRNAs microinjected into the abdominal cavity did not occur, whereas normal RNAi responses were induced when dsRNAs were expressed from the fly genome. These mutant flies also had defective antiviral immunity against Drosophila C virus (DCV), one of the RNA viruses. Interestingly, upon DCV infection, siRNAs against DCV were detected in the Ago2 complex even in clc-b or dmostm1 knockout flies. In other words, the lysosomal Cl‑/H+ antiporter ClC-b/DmOstm1 is required for systemic antiviral immune responses, rather than for siRNA production in DCV infected cells. These studies indicate that dsRNAs endocytosed and stored in vesicles have the intrinsic ability to induce ClC-b/DmOstm1-dependent LMP, allowing dsRNA translocation into the cytoplasm for RNAi responses in Drosophila cells. In addition to the systemic immune response induced by viral RNAs, this intrinsic property of dsRNA itself may contribute to various cell-to-cell communications via endocytosis.
This study was conducted in collaboration with Associate Professor Dr. Tamaki Yano, Graduate School of Pharmaceutical Sciences, Tohoku University; Professor Dr. Noriko Toyama-Sorimachi, The Institute of Medical Science, The University of Tokyo; and Associate Professor Dr. Katja Brückner (deceased), University of California, San Francisco. Four undergraduate students at the School of Pharmacy, Kumamoto University; Research Specialists Mr. Shingo Usuki, Liaison Laboratory Research Promotion Center, IMEG, Kumamoto University; and Dr. Kazuko Hanyu-Nakamura, Department of Germline Development, IMEG, Kumamoto University, also contributed to this study. This study was supported by the JSPS KAKENHI (Grant Number 22H02759 to T.Y., 21H04803 to N.T.S and 23H00364, 21H02489, and 17H03686 to A.N.), the program of the Inter-University Research Network for High Depth Omics, IMEG, Kumamoto University (to T.T. and A.N.), MEXT Promotion of Development of a Joint Usage/Research System Project: Coalition of Universities for Research Excellence Program (CURE) Grant Number JPMXP1323015486 (to A.N.), and research grants from Takeda Science Foundation and Mitsubishi Foundation (to A.N.).
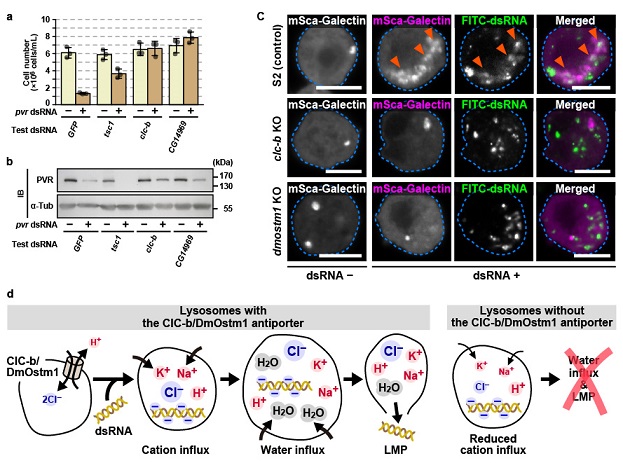
Figure Legends
(a) The number of Kc cells after culture with mixtures of dsRNAs targeting pvr and test genes (GFP, tsc1, clc-b, and CG14969). Tsc1 is a known suppressor of cell death by the depletion of PVR. The GFP dsRNA was used as a non-targeting control. (b) Immunoblots for PVR of lysates from cells cultured with mixtures of dsRNAs against pvr and test genes (GFP, tsc1, clc-b, and CG14969). Cells were cultured with dsRNAs as in a, and whole-cell lysates were prepared. α-Tubulin (α-Tub) was used as a loading control. (c) Endocytosed dsRNAs induce LMP in a ClC-b/DmOstm1-dependent manner. Representative images of mScarlet-Galectin and FITC-dsRNAs in control, clc-b, and dmostm1 KO S2 cells. mSca-Galectin+ FITC-dsRNA+ foci are indicated with arrowheads. (d) Schematic model for the dsRNA-mediated LMP process. As dsRNAs are highly negatively charged molecules, their accumulation in the lysosomes may lead to an influx of counter-ions, such as sodium and potassium ions, which are major lysosomal cations, to maintain electroneutrality. It is conceivable that the cation influx is followed by water influx because of an osmotic imbalance across the lysosomal membrane, resulting in lysosomal swelling and LMP. In the absence of the ClC-b/DmOstm1 antiporter, cation influx might be defective, causing inefficient water influx and LMP.