*Morino-Koga S., M. Tsuruda, X. Zhao, S. Oshiro, T. Yokomizo, M. Yamane, S. Tanigawa, K. Miike, S. Usuki, K. Yasunaga, R. Nishinakamura, T. Suda, *M. Ogawa. Transition of signal requirement in hematopoietic stem cell development from hemogenic endothelial cells. Proc Natl Acad Sci USA, 2024 (in press).
(* Co-corresponding authors)
Hematopoietic stem cells (HSCs) develop from hemogenic endothelial cells (HECs) in vivo during mouse embryogenesis. When cultured in vitro, cells from the embryo phenotypically defined as pre-HSC-I and pre-HSC-II have the potential to differentiate into HSCs. However, minimal factors required for HSC induction from HECs have not yet been determined.
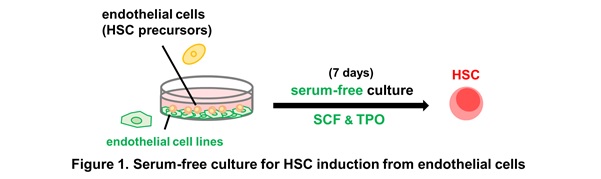
Dr. Saori Morino-Koga at the Department of Cell Differentiation (Prof. Minetaro Ogawa) demonstrated that stem cell factor (SCF) and thrombopoietin (TPO) induced engrafting HSCs from embryonic day (E) 11.5 pre-HSC-I in a serum-free and feeder-free culture condition. In contrast, E10.5 pre-HSC-I and HECs required an endothelial cell layer in addition to SCF and TPO to differentiate into HSCs (Fig.1).
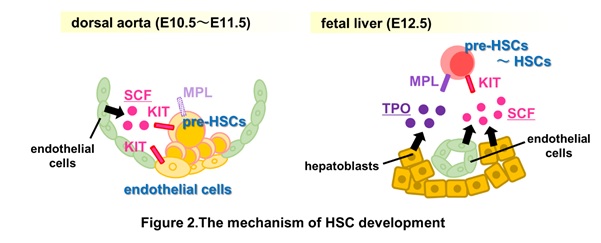
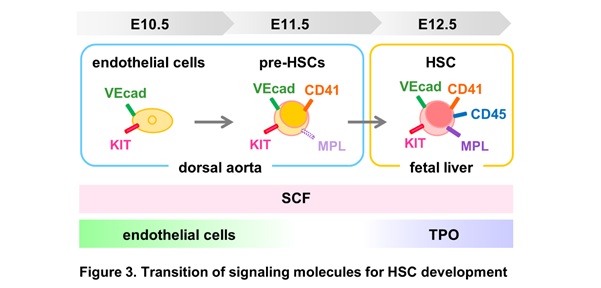
A single-cell RNA sequencing analysis of E10.5–11.5 dorsal aortae with surrounding tissues and fetal livers detected TPO expression confined in hepatoblasts, while SCF was expressed in various tissues, including endothelial cells and hepatoblasts. Our results suggest a transition of signal requirement during HSC development from HECs. The differentiation of E10.5 HECs to E11.5 pre-HSC-I in the aorta-gonad-mesonephros region depends on SCF and endothelial cell-derived factors. Subsequently, SCF and TPO drive the differentiation of E11.5 pre-HSC-I to pre-HSC-II/HSCs in the fetal liver (Fig.2 & 3).
The culture system established in this study provides a beneficial tool for exploring the molecular mechanisms underlying the development of HSCs from HECs.