
Atypical heat shock transcription factor HSF5 is critical for male meiotic prophase under non-stress conditions.
#Yoshimura S, #Shimada R, Kikuchi K, Kawagoe S, Abe H, Iisaka S, Fujimura S, Yasunaga K, Usuki S, Tani N, Ohba T, Kondoh E, Saio T, Araki K, ※Ishiguro K (# Equally contributed)
Nature Communications 15, 3330 (2024)
A group led by Professor Kei-ichiro Ishiguro and Saori Yoshimura, Ryuki Shimada of Kumamoto University’s Institute of Developmental Biology has discovered that the mechanism that controls male meiosis is essential for the production of sperm. Until now, the details of the mechanism that endures meiosis during the production of sperm had not been clarified, which may lead to future advances in fertility treatment and other reproductive medicine.
In tissues and organs throughout the body, cell proliferation usually takes place by cell division called “somatic cell division. In the ovaries and testes, on the other hand, a special type of cell division called meiosis takes place, in which the number of chromosomes is reduced to half of the original number to produce an egg or sperm (Figure 1).
During the process of oogenesis and spermatogenesis, it is known that meiosis is controlled differently in males and females. Particularly in the testes, following the completion of meiosis, there are immediate and significant morphological changes characteristic of spermatogenesis. During this process, the expression of many genes that were previously active in executing meiosis becomes inactive as they prepare for the morphological changes in sperm. However, the detailed mechanisms underlying the activation of genes involved in spermatogenesis and the progression of meiosis towards completion have remained unclear for many years, despite being crucial issues directly related to reproductive medicine such as male infertility. In this study, Professor Ishiguro’s group identified that there are different regulations between males (testes) and females (ovaries) in the mechanism of meiosis, and they further identified important genes involved in both the progression regulation of spermatogenesis specific meiosis and the activation of spermatogenesis.
In their previous research, Professor Ishiguro’s group discovered the meiosis switch gene MEIOSIN, which activates hundreds of genes involved in sperm and egg formation simultaneously, and clarified the mechanism by which MEIOSIN causes the switch from somatic cell division to meiosis(Figure 1) (Ishiguro et al., Dev Cell 2020).
In both the process of oogenesis and spermatogenesis, meiosis generally occurs through the same mechanism, but it is understood that the control differs between males and females. Particularly in the testes, significant morphological changes characteristic of spermatogenesis occur after the completion of meiosis. As spermatogenesis progresses, the expression of many genes that were previously active in executing meiosis becomes inactive. However, the detailed mechanism underlying the activation of genes involved in spermatogenesis and the progression of meiosis towards completion remains unclear. This is an important issue directly related to reproductive medicine, such as male infertility, yet it has remained an unresolved challenge worldwide for many years.
The findings of this study represent a follow-up to the discovery of MEIOSIN, a switch for meiosis, which was published by this research group in 2020. This research sheds light on the function of previously uncharacterized genes expected to act under the command of MEIOSIN. Among these, HSF5 was identified as one such gene that is expressed in spermatocytes in the testis (Figure 2). HSF5 belongs to the family of Heat Shock Factors (HSFs), proteins known to respond to heat stress. However, unlike other similar proteins such as HSF1, HSF2, HSF3, and HSF4, whose functions in response to heat stress are well known, the function of HSF5 was previously unclear.
First, through genome editing, it was discovered that when the function of the HSF5 gene in mice was abolished, male reproductive cells initially initiated meiosis but then perished midway, resulting in complete infertility (see Figure 3). Next, utilizing a technique called single-cell RNA-seq, the researchers extensively analyzed the testes of HSF5-deficient mice and found that HSF5 is essential for controlling meiosis from the mid-stage onward and is involved in activating spermatogenesis. Furthermore, using genome binding analysis, it was revealed that HSF5 exhibits activity in directly binding to DNA during the mid-stage of meiosis. Moreover, it binds to regulatory regions called promoters of many genes involved in activating spermatogenesis, thereby simultaneously completing the meiotic program and directing spermatogenesis.
Despite being classified as a Heat Shock Factor, HSF5 demonstrates activity in directly binding to DNA through a mechanism entirely different from that of previously known heat shock factors. This aspect of the discovery was academically surprising. HSF5 plays an essential role in the completion process of meiosis and is crucial for genes involved in sperm formation. Therefore, it holds potential for advancing the understanding of infertility and other reproductive medical conditions in the future.
While the findings of this study were validated using mice, it is known that HSF5 gene also exists in humans. Infertility cases in humans often have unknown causes, particularly those showing abnormalities in sperm formation. This discovery is expected to contribute to elucidating the pathology of infertility, especially cases demonstrating sperm formation abnormalities. Moreover, given the recent trend of delayed marriages and the initiation of insurance coverage for infertility treatments, there is a societal background that anticipates significant contributions to reproductive medicine in the future. This includes the development of technologies to ensure the quality of meiosis, among other applications.
[Fundings]
Grant from Japan Agency for Medical Research and Development (AMED) JP21m6310021
Grant-in-Aid for Scientific Research on Innovative Areas from MEXT #19H05743, #23H00379, #22K19315
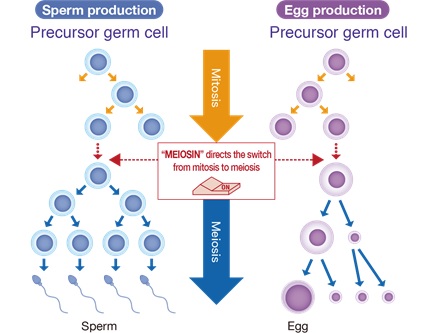
Figure 1. The Meiosin initiates the switch from mitosis to meiosis
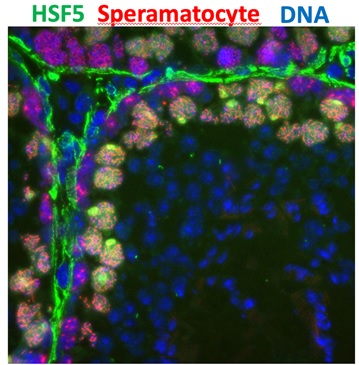
Figure 2. Specific expression of HSF5 in spermatocytes in the testis tubules
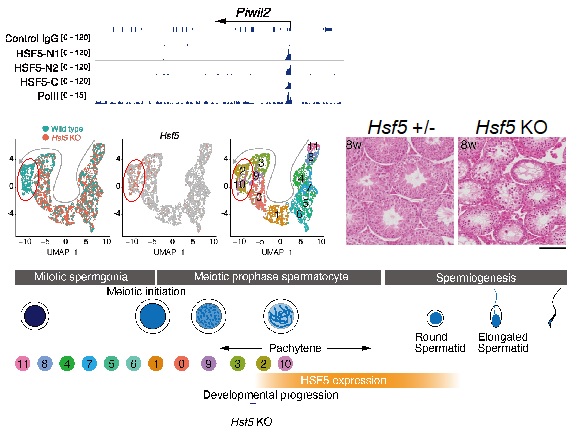
Figure3. HSF5 dplays a crucial role in the progression of male meiosis