
STRA8–RB interaction is required for timely entry of meiosis in mouse female germ cells
Ryuki Shimada, Yuzuru Kato, Naoki Takeda, Sayoko Fujimura, Kei-ichiro Yasunaga, Shingo Usuki, Hitoshi Niwa, Kimi Araki, Kei-ichiro Ishiguro
Nature Communications DOI:10.1038/s41467-023-42259-6
A group led by Professor Kei-ichiro Ishiguro and Assistant Professor Ryuki Shimada of Kumamoto University’s Institute of Developmental Biology has discovered that the mechanism that controls meiosis, a process necessary for the formation of oocytes, is essential to unlock the function of tumor suppressor proteins. Until now, the details of the mechanism that causes meiosis during egg production had not been clarified, which may lead to future advances in fertility treatment and other reproductive medicine.
In tissues and organs throughout the body, cell proliferation usually takes place by cell division called Mitosis. In the ovaries and testes, on the other hand, a special type of cell division called meiosis takes place, in which the number of chromosomes is reduced to half of the original number to produce an egg or sperm (Figure 1). In males, meiosis is repeated in the testes almost throughout life after puberty to produce sperm. In contrast, meiosis is initiated in the ovary during a very limited period of time during the fetal period (inside the pregnant mother) in the female. Once the egg enters meiosis during the fetal period, it enters a prolonged state of dormancy until ovulation occurs (Figure 2). Thus, unlike the male, the only chance for the female germ cell to enter meiosis and become an egg is during the fetal period, long before birth. The germ cells that are able to enter meiosis during the limited time of fetal life in the female ovary determine the number of reproductive eggs needed throughout life. However, the mechanism specific to women who enter meiosis only during a certain period of fetal life was not understood at all. In this study, we found that the meiosis initiation mechanism is regulated differently in males (testes) and females (ovaries), and that the ovary-specific mode of meiosis initiation involves the function of a tumor suppressor protein.
In their previous research, Professor Ishiguro’s group discovered the meiosis switch gene MEIOSIN, which activates hundreds of genes involved in sperm and egg formation simultaneously, and clarified the mechanism by which MEIOSIN causes the switch from somatic cell division to meiosis (Ishiguro et al., Dev Cell 2020).
Especially in females, the switch from somatic cell division to meiosis occurs only during a very limited period of time before birth, while the germ cells arestill in the mother’s womb, so the details of the mechanism are unknown and have not been well studied. The control of meiosis in women is also an important issue that is directly related to infertility and other reproductive medical problems such as primary ovarian failure (premature menopause), yet it has been an issue that has remained unexplored worldwide for many years.
In order to investigate in detail how and when meiosis occurs during the egg production process, Professor Ishiguro’s group created genetically engineered mice and conducted research to analyze the proteins contained in the ovaries and to examine the functions of various genes. Using mass spectrometry, they found that a protein called STRA8 binds to retinoblastoma protein (RB), a tumor suppressor protein. It was already known that retinoblastoma protein (RB) acts as a brake on cell proliferation by moderately suppressing DNA synthesis to prevent cells from proliferating too fast. In addition, the group’s previous research had shown that STRA8 protein binds to MEIOSIN, which acts as a “switch” for meiosis, but why it binds to RB was a mystery.
Therefore, it was found that when the binding between STRA8 and RB was eliminated by introducing artificial mutations into the mouse STRA8 gene through genome editing, the eggs were depleted early and infertility resulted (Figure 3). Furthermore, using the single-cell RNA-seq method, an analytical technique that detects gene expression in a small number of cells, they examined the behavior of various genes and found that meiosis no longer occurs in the female ovary at the appropriate time when it should occur in the fetal period. Essentially, in order for meiosis to occur, the genome must have undergone a prior process of DNA synthesis to undergo genome doubling. However, when the STRA8 protein fails to bind to RB, RB continues to function strongly in the female mouse germ cells, and the DNA synthesis process of genome doubling does not function properly at the appropriate time, which further results in the failure of MEIOSIN activation The results of the study showed that the RBs were not working properly at the right time in the mutant mice. As a result, meiosis is not initiated at the proper time as planned (Figure 4).
They found that most oocytes die early before and after birth due to the induction of the apoptosis gene, and that the storage of oocytes in the ovary fails (Figure 5). From these series of studies, they have elucidated that the STRA8 protein transiently relaxes RB function to promote DNA synthesis, which is an important mechanism for triggering meiosis in the fetal ovary during the oocyte formation.
Although these results were verified using mice, it is suggested that this mechanism exists in all mammals, including humans. It is known that there are many cases of infertility in humans for which the cause is unknown. This discovery is expected to contribute to the elucidation of the pathophysiology of infertility, such as primary ovarian failure and premature menopause, which are indicative of inadequate oocyte formation and early depletion.
In the case of women, the number of germ cells that begin meiosis during the fetal period in the mother’s body during pregnancy determines the number of eggs that will be stored for future adulthood. In other words, before a woman is born, meiosis has been initiated and a lifetime supply of eggs has been prepared. This may provide a guideline for medication that may affect the tumor suppressor protein RB during pregnancy and in drug discovery.
In the course of this research, they further found that genes whose functions have not been fully analyzed are regulated by the release of RB via the STRA8 protein. In the future, elucidation of the function of these genes during the oocyte formation process is expected to contribute greatly to reproductive medicine.
[Fund(s)]
Grant from Japan Agency for Medical Research and Development (AMED) JP21m6310021
Grant-in-Aid for Scientific Research on Innovative Areas from MEXT #19H05743, #23H00379, #22K19315
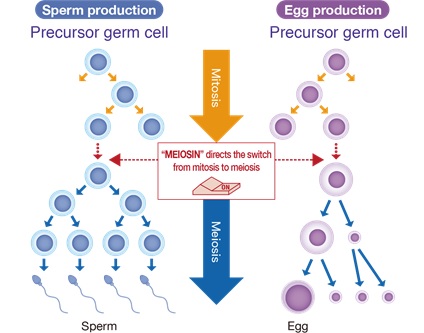
Figure 1 : The “Meiosin” initiates the switch from mitosis to meiosis
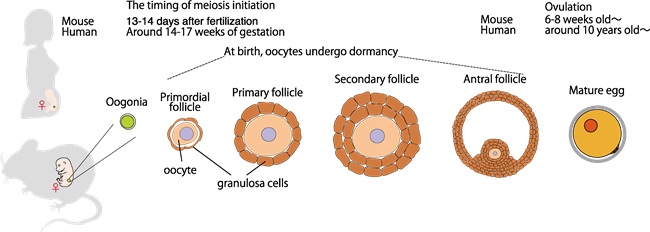
Figure 2 : Meiotic initiation, dormancy, and maturation processes of oocyte