
Koji Kikuchi*, Yasuhisa Sakamoto, Akiyoshi Uezu, Hideyuki Yamamoto, Kei-ichiro Ishiguro, Kenji Shimamura, Taro Saito, Shin-ichi Hisanaga, and Hiroyuki Nakanishi (*, corresponding author)
Map7D2 and Map7D1 facilitate microtubule stabilization through distinct mechanisms in neuronal cells
Life Sci. Alliance
DOI: 10.26508/lsa.202201390
A group led by Dr. Koji Kikuchi, a lecturer at Department of Molecular Pharmacology, Graduate School of Medical Sciences, Kumamoto University (will join Department of Chromosome Biology, IMEG from 1st May) has discovered a new mechanism that stabilizes microtubules to control cell morphology and motility of neuronal cells
Cells take the appropriate morphology to accomplish cellular functions. And it is known that cell morphology is controlled by the cytoskeletal dynamics. Microtubules, one of the cytoskeletons, are dynamically elongated and shortened by polymerization and depolymerization of tubulin. Such microtubule dynamics are essential to accomplish cellular functions with morphological changes such as cell motility and neurite outgrowth. Although various molecules involved in microtubule dynamics have been identified, the regulatory mechanisms of microtubule dynamics are diverse depending on the cellular functions and remain unclear.
Our research aims to understand the regulatory mechanisms of microtubule dynamics in various cellular functions (EMBO J., 2010.; J. Cell Sci., 2012.; EMBO Rep., 2018.). Based on our previous studies, we focused on Map7D2, which belongs to a family of microtubule-associated proteins called the MAP7 family. We found that Map7D2 controls cell motility and neurite outgrowth of mouse N1-E115 neuronal cells by binding to and directly stabilizing microtubules. In addition, we found that Map7D1, which like Map7D2 belongs to the MAP7 family, exhibits similar subcellular localization and gene knock-down phenotypes to Map7D2. However, in contrast to Map7D2, Map7D1 is required for the maintenance of acetylated stable microtubules. Thus, although Map7D2 and Map7D1 belong to the same family, they stabilize microtubules through distinct mechanisms to control cell motility and neurite outgrowth, indicating the existence of functional diversity among the families. Furthermore, we analyzed the expression distribution of Map7D2 in various organs in rodents and found that Map7D2 is expressed predominantly in the brain and testis. Interestingly, Map7D2 is the most highly expressed in the Map2-negative area of the olfactory bulb, i.e., the glomerular layer where the axons of olfactory neurons accumulate. Analysis of the expression distribution suggests that Map7D2 may regulate neuronal morphology in vivo by regulating the microtubule stabilization.
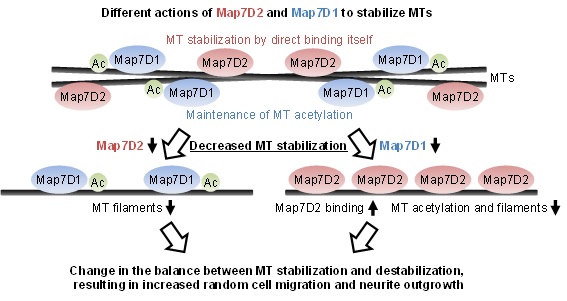
Figure: Proposed model for the distinct mechanisms of Map7D2 and Map7D1 for MT stabilization in neuronal cells.