
Intraembryonic hemogenic endothelial cells form mast cells independently of hematopoietic stem cell development
Mariko Tsuruda, Saori Morino-Koga, Minetaro Ogawa
Hematopoietic Stem Cell-Independent Differentiation of Mast Cells From Mouse Intraembryonic VE-Cadherin+ Cells
Stem Cells. 2022: doi: 10.1093/stmcls/sxac001.
Intraembryonic hemogenic endothelium not only forms hematopoietic stem cells (HSCs) but also generates tissue-resident blood cells in a stem cell-independent manner. Mast cells (MCs) are proposed to originate from intraembryonic hemogenic endothelium, although it is obscure whether the MC development is stem cell-dependent or not. We demonstrate that intraembryonic hemogenic endothelial cells (HECs) differentiate into MCs without forming stem cells in culture and contribute to intraperitoneal MCs after transplantation into newborn hosts. Our findings put MCs on the list of blood cells that develop from intraembryonic HECs via stem cell-independent pathway.
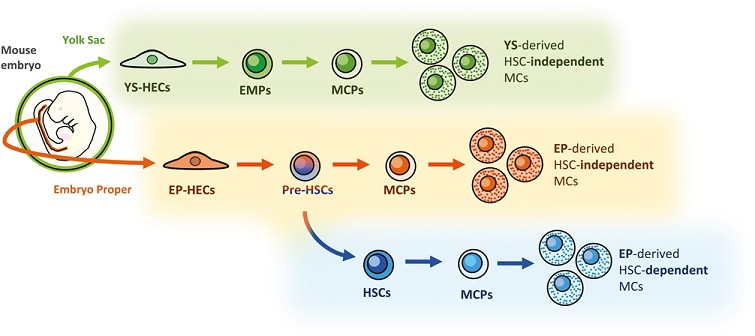
Figure: Three developmental pathways of MCs in the mouse embryo. VE-cadherin+ CD45− HECs derived from the yolk sac and embryo proper can differentiate into MCs independently of HSC development. Besides, HECs in the embryo proper differentiate into HSCs, from which an HSC-dependent MC lineage can be derived.
Summary of the findings
Hematopoietic stem cell (HSC)-independent hematopoiesis from hemogenic endothelial cells (HECs) in the mouse embryo has been recognized as a source of tissue-resident hematopoietic cells in adult mice. Connective tissue mast cells (MCs) have been reported to originate from VE-cadherin (VE-cad)-expressing HECs in the yolk sac and embryo proper (EP) by a VE-cad-Cre-mediated lineage-tracing analysis. However, it remains unclear whether MCs are generated via a conventional HSC-dependent hematopoietic differentiation pathway, or whether through a fast-track pathway bypassing the emergence of HSCs.
Here, we investigated whether EP-derived VE-cad+ cells differentiate into MCs independently of HSCs. VE-cad+ cells isolated from the embryonic day (E) 9.5-10.5 EP robustly formed connective tissue-type MCs in a newly established co-culture system using PA6 stromal cells. In contrast, bone marrow (BM) reconstitution assays of cultured cells indicated that E9.5 VE-cad+ cells did not differentiate into transplantable HSCs in this culture condition. Lymphoid-biased HSCs with a limited self-renewal capacity were occasionally detected in some cultures of E10.5 VE-cad+ cells, while MC growth was constantly observed in all cultures examined. HSCs purified from adult BM required a more extended culture period to form MCs in the PA6 co-culture than the embryonic VE-cad+ cells. Furthermore, E9.5-E10.5 VE-cad+ cells contributed to tissue-resident MCs in postnatal mice when transplanted into the peritoneal cavity of newborn mice.
These results suggest that EP-derived VE-cad+ cells generate MCs independently of HSC development in vitro and possess the potential of generating connective tissue MCs in vivo.