
Renyong Guo, Hiroshi Sakamoto, Shigeki Sugiura and Minetaro Ogawa (2007) Endothelial cell motility is compatible with junctional integrity. J. Cell. Physiol. 211, 327-335.
Confluent endothelial cells in culture are generally regarded as a model of resting endothelium in blood vessels (i.e., forming junctions at points of cell-cell contact, losing ability to proliferate in response to growth factors, and remaining stationary). However, incompatibility between junctional integrity and endothelial cell motility remains uncertain. The aim of this study was to determine whether endothelial cells (in colonies generated from differentiating embryonic stem cells in contact with OP9 stromal cell layer) have a resting endothelial phenotype (i.e., lack motility). Time-lapse analyses showed that though endothelial cells were connected to each other through adherens junctions and tight junctions, they were moving continuously within the colonies. Endothelial cell movement was accompanied by formation of lamellipodia, which transiently accumulated green fluorescent protein-tagged β -actin and p41-Arc (a subunit of the actin-related protein 2/3 complex) at their anterior tips, suggesting that the movement is an active behavior of endothelial cells. Endothelial cell-specific expression of yellow fluorescent protein-tagged vascular endothelial-cadherin and claudin-5 revealed that adherens junctions and tight junctions persisted during endothelial cell migration. Furthermore, intercellular junctions underwent dynamic remodeling at the leading edge of moving endothelial cells. These results suggest that endothelial cells can remain highly motile without losing intercellular junctions.
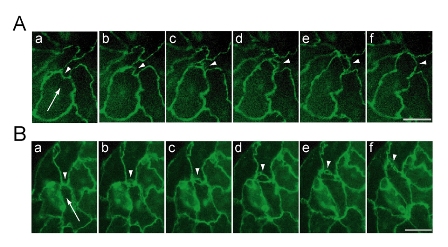
Figure: Remodeling of intercellular junctions at the leading edge of moving endothelial cells. Time-lapse analyses of endothelial cell colonies expressing VE-cadherin-YFP (A) or YFP-claudin-5 (B) was performed under a fluorescence microscope equipped with shutter device and CCD camera. YFP fluorescence images were acquired at intervals of 5 minutes, and six frames of every 10 minutes were extracted for display (a-f). Arrows point to the direction of cell movement. Arrowheads indicate the protrusion of leading edge. Scale bars indicate 25 μ m.