
Yuki Murayama, Teru Ogura, Kunitoshi Yamanaka (2015) Characterization of C-terminal adaptors, UFD-2 and UFD-3, of CDC-48 on the polyglutamine aggregation in C. elegans. Biochem. Biophys. Res. Commun. 459: 154-160
CDC-48 (also called VCP or p97 in mammals and Cdc48p in yeast) is a AAA (ATPases associated with diverse cellular activities) chaperone and participates in a wide range of cellular activities including modulation of protein complexes and protein aggregates. UFD-2 and UFD-3, C-terminal adaptors for CDC-48, reportedly bind to CDC-48 in a mutually exclusive manner and they may modulate the fate of substrates for CDC-48. However, their cellular functions have not yet been elucidated. In this study, we found that CDC-48 preferentially interacts with UFD-3 in Caenorhabditis elegans. We also found that the number of polyglutamine (polyQ) aggregates was reduced in the ufd-3 deletion mutant but not in the ufd-2 deletion mutant (Figure). Furthermore, the lifespan and motility of the ufd-3 deletion mutant, where polyQ40::GFP was expressed, were greatly decreased. Taken together, we propose that UFD-3 may promote the formation of polyQ aggregates to reduce the polyQ toxicity in C. elegans.
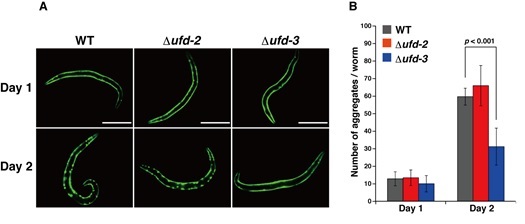
Figure. (A) Fluorescence micrographs of deletion mutants of UFD-2 and UFD-3 expressing polyQ40::GFP in body wall muscle cells. (B) The number of polyQ40::GFP aggregates of each mutant as shown in (A) was counted.