
Ola Shalaby, Tomoko Ohmori, Koichiro Miike, Shunsuke Tanigawa, Luh Ade Wilan Krisna, Alessia Calcagnì, Andrea Ballabio, Yoshiaki Kubota, Laura S. Schmidt, W. Marston Linehan, Takaaki Ito, Masaya Baba, Ryuichi Nishinakamura. Folliculin deletion in the mouse kidney results in cystogenesis of the loops of Henle via aberrant TFEB activation. The American Journal of Pathology, online (2025)
Folliculin (FLCN) is a causative gene for Birt-Hogg-Dubé (BHD) syndrome, which is characterized by a variety of manifestations including renal cysts and cancer. We report here that nephron-specific Flcn knockout mice exhibit cystogenesis along the entire nephron segments, most prominent in the loop of Henle (LoH). Single-cell RNA sequencing revealed many upregulated genes, especially in the knockout LoH. These genes include those related to lysosomal activity and mTORC1 activation and are likely targets of TFE3/TFEB. The double Flcn/Tfeb knockout largely reverses most of the phenotypes along the entire nephron. Our findings reveal the essential role of the FLCN-TFEB signaling pathway in nephron development, particularly in LoH, and shed light on the pathogenesis of BHD syndrome.
Birt-Hogg-Dubé (BHD) syndrome is an autosomal dominant syndrome characterized by renal cysts and tumors, benign skin papules, lung cysts and spontaneous pneumothorax. Folliculin (FLCN) was identified as a novel causative tumor suppressor gene in patients with BHD syndrome.
The kidney contains many nephrons, each consisting of a glomerulus, proximal tubule, loop of Henle (LoH), and distal tubule. LoH has a unique loop structure that runs longitudinally along the corticomedullary axis and is essential for the production of hypertonic urine.
Here we generated nephron-specific Flcn knockout mice and found numerous cysts in all nephron segments, which were most pronounced in LoH. The knockout mice died shortly after birth and those with milder phenotypes survived. Single-cell RNA sequencing revealed many upregulated genes, especially in the knockout LoH. These genes included those related to lysosomal activity and mTORC1 activation, which are likely targets of TFE3/TFEB. While the double Flcn/Tfe3 knockout only ameliorated the glomerular cysts, the double Flcn/Tfeb knockout largely reversed most of the phenotypes throughout the nephron. Thus, Flcn deletion leads to cystogenesis via aberrant TFEB activation (Figure).
These results reveal the essential role of the FLCN-TFEB signaling pathway in nephron development, particularly in LoH, and shed light on the pathogenesis of BHD syndrome. TFEB inhibitors may be a promising treatment for BHD patients. Human kidney organoids lacking FLCN could also be useful in examining the efficacy of such a treatment.
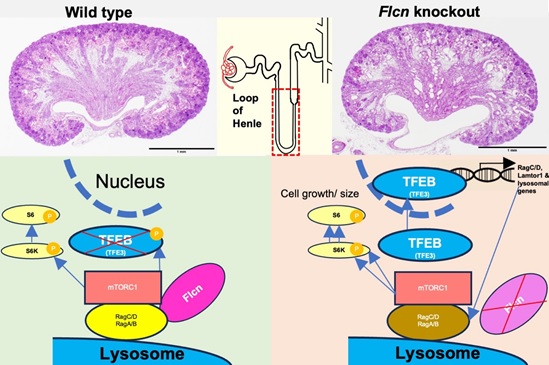
Figure. The Flcn-TFEB signaling pathway is responsible for cyst formation in the kidney.
Under normal conditions, Flcn acts as a GTPase-activating protein (GAP) that activates the RagC/D and RagA/B complex. This complex facilitates the recruitment of mTORC1 to the surface of the lysosome. mTORC1 is then activated and phosphorylates many substrates, including S6 kinase. It also phosphorylates TFEB, leading to its inactivation. However, in the absence of Flcn, RagC/D remains inactive and cannot phosphorylate TFEB via mTORC1. Unphosphorylated TFEB then translocates to the nucleus, where it activates transcription of its target genes, including RagC/D, Lamtor1, and those related to lysosomal biogenesis. This causes paradoxical hyperactivation of mTORC1, which leads to excessive cell growth and cyst formation. Modified from the paper by Shalaby et al. described above.