
Title: RNA G-quadruplexes form scaffolds that promote neuropathological α-synuclein aggregation.
Kazuya Matsuo, Sefan Asamitsu, Kohei Maeda, Hiroyoshi Suzuki, Kosuke Kawakubo, Ginji Komiya, Kenta Kudo, Yusuke Sakai, Karin Hori, Susumu Ikenoshita, Shingo Usuki, Shiori Funahashi, Hideki Oizumi, Atsushi Takeda, Yasushi Kawata, Tomohiro Mizobata, Norifumi Shioda* and Yasushi Yabuki*
(* Co-corresponding authors)
Cell
doi:10.1016/j.cell.2024.09.037
URL:https://doi.org/10.1016/j.cell.2024.09.037
A research group led by Professor Norifumi Shioda, Yasushi Yabuki, Kazuya Matsuo and their colleagues in Department of Genomic Neurology, Institute of Molecular Embryology and Genetics (IMEG), Kumamoto University has newly elucidated the pathogenic mechanism of synucleinopathy.
As a consequence of the ageing of society, the number of patients with neurodegenerative diseases is increasing rapidly, thereby rendering the development of countermeasures an urgent priority. Synucleinopathies (e.g. Parkinson’s disease, Dementia with Lewy Body, multiple system atrophy) and tauopathies (e.g. Alzheimer’s disease) are caused by the aggregation, accumulation and intercellular transmission of abnormal proteins in the brain, leading to a loss of neuronal function and symptoms such as cognitive and motor impairment. In synucleinopathy, proteins called “α-synuclein” aggregate in the cells, causing neurological damage. However, the mechanism by which α-synuclein, which does not typically form aggregates, undergoes aggregation within the cell, remained unclear.
Our research group has successfully identified a molecule that aggregates α-synuclein intracellularly. It was an RNA structure called ‘G-quadruplex; G4’*. The upregulation and assembly of G4 structures has been observed in stress-induced cells (1), and the assembly of G4 has been identified as a scaffold for the aggregation of α-synuclein. Indeed, post-mortem analysis of the brains in patients with Parkinson’s disease revealed the accumulation of G4 in approximately 90% of α-synuclein aggregates. When G4 was artificially assembled in neurons in the mouse brain, intracellular α-synuclein co-aggregated with G4 as a scaffold, causing neurodegeneration and motor dysfunction. Furthermore, oral administration of 5-aminolevulinic acid (2), a drug found by our research group to inhibit G4 assembly, inhibited α-synuclein aggregation and prevented progressive loss of motor function seen in synucleinopathy model mice.
In this study, we identified for the first time that the molecule responsible for the intracellular aggregation of α-synuclein is “G4.” Furthermore, we demonstrated that the suppression of G4 assembly can effectively prevent the onset of synucleinopathy. Our research group has previously reported that G4 assembly also causes neurodegeneration in cases of hereditary neurodegenerative disease (3). Furthermore, our findings also revealed that a protein known as “Tau,” which is intricately linked to Alzheimer’s disease, undergoes aggregation in response to G4 (4). Thus, suppressing “G4 assembly” will lead to drug discovery for “pre-symptomatic state” neurodegenerative diseases in general.
[Funding]
This work was supported by AMED (grant numbers: JP21wm0525023 and JP21gm6410021 to Y.Y.), JSPS KAKENHI (grant numbers: JP21K20723 and JP22J00687 to K.Mat; JP21K06579 and JP23H03851 to Y.Y.; and JP21H00207, JP20K21400, JP22K19297 and JP23H00373 to N.S.), JST FOREST Program (JPMJFR2043 to N.S.), MEXT Promotion of Development of a Joint Usage/Research System Project: Coalition of Universities for Research Excellence Program (CURE) (JPMXP1323015486), The Pharmacological Research Foundation, Tokyo (to Y.Y.), Kowa Life Science Foundation (to Y.Y.), Narishige Neuroscience Research Foundation (to Y.Y.), the Mochida Memorial Foundation for Medical and Pharmaceutical Research (to N.S.), the Astellas Foundation for Research on Metabolic Disorders (to N.S.), the Foundation of SBI Pharmaceuticals Co., Ltd. (to N.S.), Takeda Science Foundation (to N.S.), International Research Core for Stem Cell-based Developmental Medicine Encourage Project, and The Inter-University Research Network for High Depth Omics, Institute of Molecular Embryology and Genetics, Kumamoto University.
[Reference]
1. Asamitsu et al. Science Advances 9, eade2035. (2023)
2. Shioda et al. Nature Medicine 24, 802-813. (2018)
3. Asamitsu et al. Science Advances 7, eabd9440. (2021)
4. Yabuki et al. bioRχiv doi: https://doi.org/10.1101/2024.03.01.582861
*G-quadruplex (G4): One of types of structure observed in both DNA and RNA. G4 is formed by guanine-rich nucleic acid sequences. G4 structure is constituted by two or more overlapping planes (G-quartet), in which four guanines form a tetramer (see figure below). In this study, we have demonstrated that the assembly of G4 formed by RNA serves as a scaffold for α-synuclein aggregation.
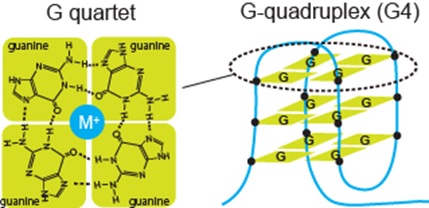
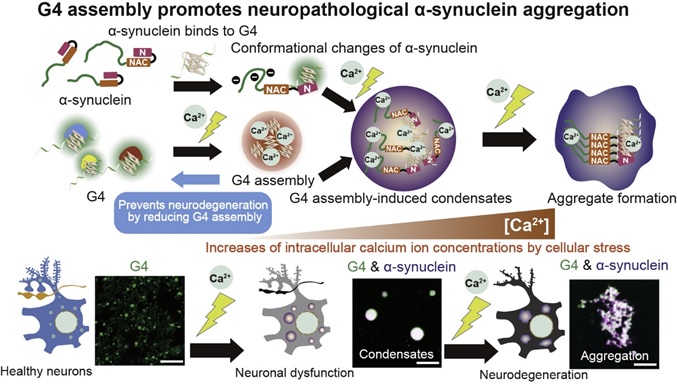
Figure legend
Neurodegeneration is triggered by the assembly of G4 associated with increased intracellular Ca2+ concentrations due to cellular stress. In addition, α-synuclein binds directly to G4 and converts its conformation to an aggregative form. α-synuclein forms aggregates following the assembly of G4 as a scaffold. Therefore, suppression of G4 assembly leads to suppression of α-synuclein aggregation, which can prevent the neurological dysfunction.