
Shin-ichiro Takebayashi, Hiroshi Tanaka, Shinjiro Hino, Yuko Nakatsu, Tomoka Igata, Akihisa Sakamoto, Masashi Narita, and Mitsuyoshi Nakao. Retinoblastoma protein promotes oxidative phosphorylation through up-regulation of glycolytic genes in oncogene-induced senescent cells. Aging Cell 14, 689-697 (2015).
Metabolism is closely linked with cellular state and biological processes, but the mechanisms controlling metabolic properties in different contexts remain unclear. Cellular senescence is an irreversible growth arrest induced by various stresses, which exhibits active secretory and metabolic phenotypes. Here, we show that retinoblastoma protein (RB) plays a critical role in promoting the metabolic flow by activating both glycolysis and mitochondrial oxidative phosphorylation (OXPHOS) in cells that have undergone oncogene-induced senescence (OIS) (Figure). A combination of real-time metabolic monitoring, and metabolome and gene expression analyses showed that OIS-induced fibroblasts developed an accelerated metabolic flow. The loss of RB downregulated a series of glycolytic genes and simultaneously reduced metabolites produced from the glycolytic pathway, indicating that RB upregulates glycolytic genes in OIS cells. Importantly, both mitochondrial OXPHOS and glycolytic activities were abolished in RB-depleted or downstream glycolytic enzyme-depleted OIS cells, suggesting that RB-mediated glycolytic activation induces a metabolic flux into the OXPHOS pathway. Collectively, our findings reveal that RB essentially functions in metabolic remodeling and the maintenance of the active energy production in OIS cells.
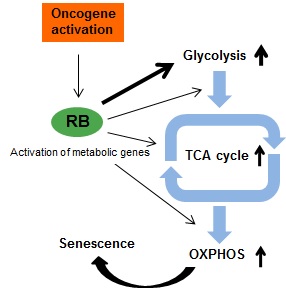
Figure Schematic model for RB-mediated activation of both glycolysis and mitochondrial OXPHOS in OIS cells.
In response to oncogenic signals, RB either directly or indirectly upregulates the mRNA levels of target genes such as glycolytic genes. Glycolytic stimulation promotes a metabolite flux into the TCA cycle, leading to OIS-driven mitochondrial OXPHOS activation. Consequently, OIS cells are metabolically activated under growth arrest, compared with normal cells and proliferative cancer cells.