FBXO47 is essential for preventing the synaptonemal complex from premature disassembly in mouse male meiosis
Nobuhiro Tanno#, Kazumasa Takemoto#, Yuki Takada-Horisawa, Ryuki Shimada, Sayoko Fujimura, Naoki Tani, Naoki Takeda, Kimi Araki and Kei-ichiro Ishiguro
(# These authors equally contributed)
iScience 25,104008 (2022)
DOI: 10.1016/j.isci.2022.104008
A research group from the Institute of Molecular Embryology and Genetics at Kumamoto University, Japan has discovered a gene, “FBXO47”, that plays a critical role in meiosis. The gene appears to be related to the cause of male infertility and could be a big step forward for reproductive medicine.
Meiosis is a specialized cell division that generates sperm or egg. During Meiosis, genetic information is exchanged between maternal and paternal chromosomes by meiotic recombination, which gives genetic differences to the next generation. Homolog chromosome synapsis is crucial for meiotic recombination. In this study, Drs. Ishiguro, Tanno and Takemoto discovered a novel gene that plays a crucial role in stabilizing homolog chromosome synapsis during meiosis.
Previously, the same group discovered Meiosin gene(Ishiguro et al., Dev Cell 2020), that acts as the switch that turns on meiosis, the special type of cell division that creates eggs and sperm, and this includes the turning-on of hundreds of other genes in the process. However, the functions of those genes have not yet been fully elucidated. “Fbxo47” gene was one of those genes controlled by MEIOSIN, but its function was unknown.
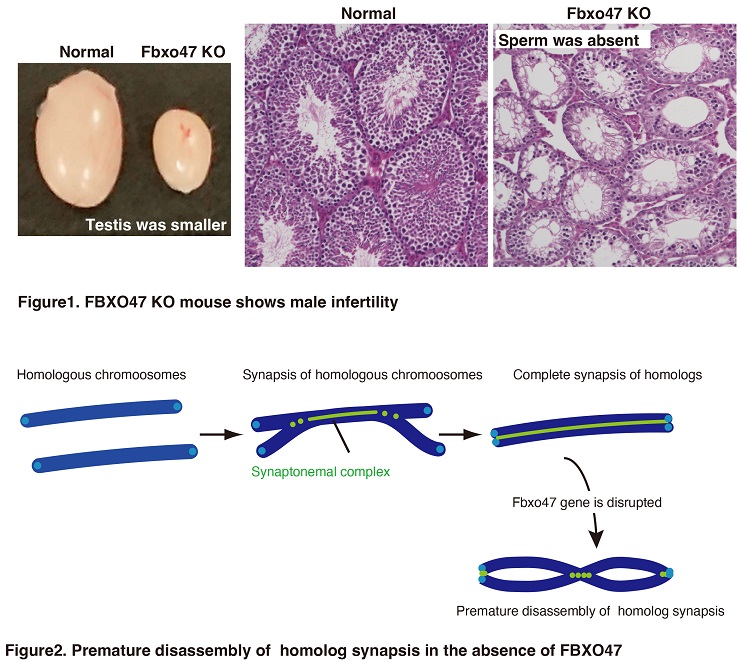
Recently, they set out to assess “Fbxo47” gene to clarify its role in meiosis. In animal experiments using genome editing technology, the researchers found that male mice became infertile if the Fbxo47 gene was artificially inhibited. Further analysis of the mouse male gonads clarified that the gene plays an essential role in stabilizing homolog synapsis. Disruption of FBXO47 shows severe impact on homologous chromosome synapsis, meiotic recombination and XY body formation, leading to male infertility (Figure 1). Notably, in the absence of FBXO47, although once homologous chromosomes are synapsed, the synaptonemal complex is precociously disassembled before progressing beyond pachytene (Figure 2). Remarkably, Fbxo47 KO spermatocytes remain in earlier stage of meiotic prophase I and lack crossovers. Ishiguro lab proposes that FBXO47 plays a crucial role in preventing synaptonemal complex from premature disassembly during cell cycle progression of meiotic prophase I.
There are many cases of human male infertility where the cause is unknown; this finding potentially reveals a new pathology. Even though these experiments were performed on animal models, the Fbxo47 gene is present in humans. Therapies and diagnosis developed from this research could ensure meiosis quality and decrease the instances of these complications.