
Naoki Ohtsu, Ikuo Nobuhisa, Miyuki Mochita, and Tetsuya Taga (2007) Inhibitory effects of homeodomain-interacting protein kinase 2 on the aorta–gonad–mesonephros hematopoiesis. Exp. Cell Res. 313, 88-97.
Definitive hematopoiesis starts in the aorta-gonad-mesonephros (AGM) region of the mouse embryo. Our previous studies revealed that STAT3, a gp130 downstream transcription factor, is required for AGM hematopoiesis and that homeodomain-interacting protein kinase 2 (HIPK2) phosphorylates serine-727 of STAT3. HIPK2 is a serine/threonine kinase known to be involved in transcriptional repression and apoptosis. In the present study, we examined the role of HIPK2 in hematopoiesis in mouse embryo. HIPK2 transcripts were found in fetal hematopoietic tissues such as the mouse AGM region and fetal liver. In cultured AGM cells, HIPK2 protein was detected in adherent cells. Functional analyses of HIPK2 were carried out by introducing wild-type and mutant HIPK2 constructs into AGM cultures. Production of CD45 + hematopoietic cells was suppressed by forced expression of HIPK2 in AGM cultures. This suppression required the kinase domain and nuclear localization signals of HIPK2, but the kinase activity was dispensable. HIPK2-overexpressing AGM-derived nonadherent cells did not form hematopoitic colonies in semisolid medium. Furthermore, overexpression of HIPK2 in AGM cultures impeded the expansion of CD45 low c-Kit + cells, which exhibit the immature hematopoietic progenitor phenotype. These data indicate that HIPK2 plays a negative regulatory role in AGM hematopoiesis in the mouse embryo.
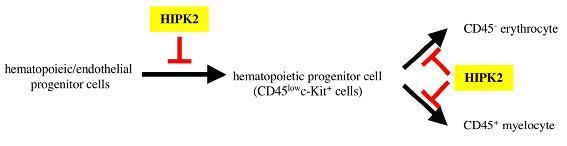
Figure: Our data suggested that HIPK2 inhibited differentiation of hematopoietic/endothelial progenitor cells into hematopoietic progenitor cells in AGM hematopoiesis. In addition HIPK2 inhibited differentiation of hematopoietic progenitor cells into macrophage, granulocyte, and erythrocyte.