Our body, organs, and tissues are well designed for their functions. How do we obtain these forms? To answer this question, we study morphogenesis using Xenopus embryos and larvae, some of the powerful models in developmental biology.
We are interested in the collective cell movement during morphogenesis. Imaging cell behavior, analyzing their movements, and identifying the regulatory genes will answer the above question. In addition to gene function, the environment and external stressors, such as nutrient unavailability, physical threats, and fluctuating temperature, can manipulate the process of morphogenesis. However, developing animals have a unique ability to overcome external stresses based on their active gene expression for development. In our laboratory, we aim to find the flexible and resilient systems underlying the cellular responses against external stressors that can disturb the processes of morphogenesis.
Thyroid morphogenesis and external nutrients
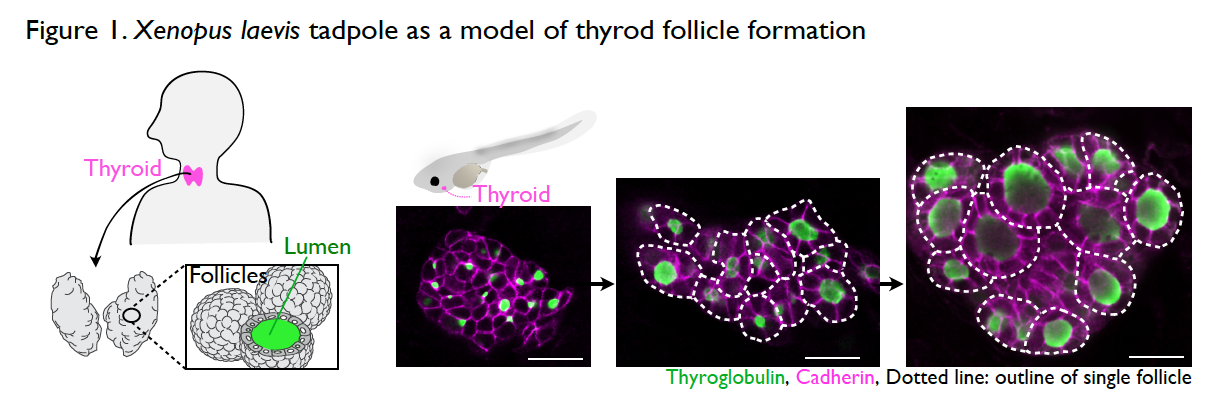
It is still a mystery as to how the thyroid gland is formed during development. The thyroid is an endocrine organ with spherical, single-layer follicles that secrete the thyroid hormone. The thyroid follicle morphology is well conserved among vertebrates including Xenopus. The process of thyroid follicle formation during organogenesis can be well observed in Xenopus tadpoles (Fig. 1).
Interestingly, thyroid organogenesis is arrested or resumed by the absence or presence of external nutrient sources (Fig. 2). Unlike mammalian embryos in utero, during organogenesis, amphibians obtain nutrients from food after mouth development. This unique part of the life cycle enables us to test the role of nutrients in organogenesis, simply by changing nutrient availability. We try to understand the underlying mechanisms of thyroid morphogenesis by focusing on nutrient-dependent remodeling of the cytoskeleton, cell adhesion, and cell polarization.
The study of nutrient-dependent thyroid development will lead us to focus on the morphogenesis of multiple organs to clarify the inter-organ communications. In the future, identifying the mechanisms of systemic regulation will not only contribute to advancing our understanding of organogenesis but also assist in understanding the pathology of congenital diseases such as thyroid deficiencies.
Cytoskeletal regulations for epithelial homeostasis and wound closure
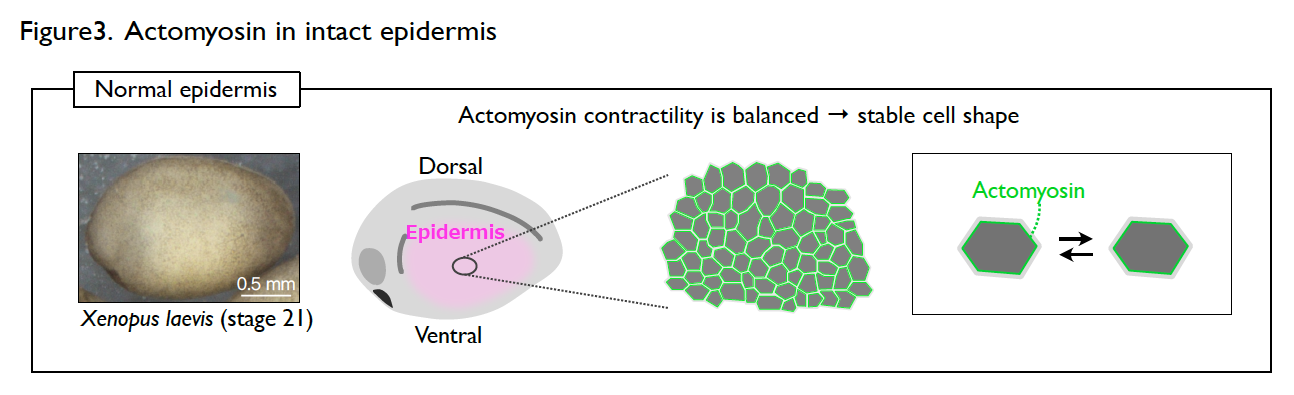
The developing epidermis of Xenopus embryos has a stable cell shape and rarely shows dynamic collective cell movement. However, the cells immediately become active once the epidermis is wounded. How the cells switch their behavior in response to wounding?
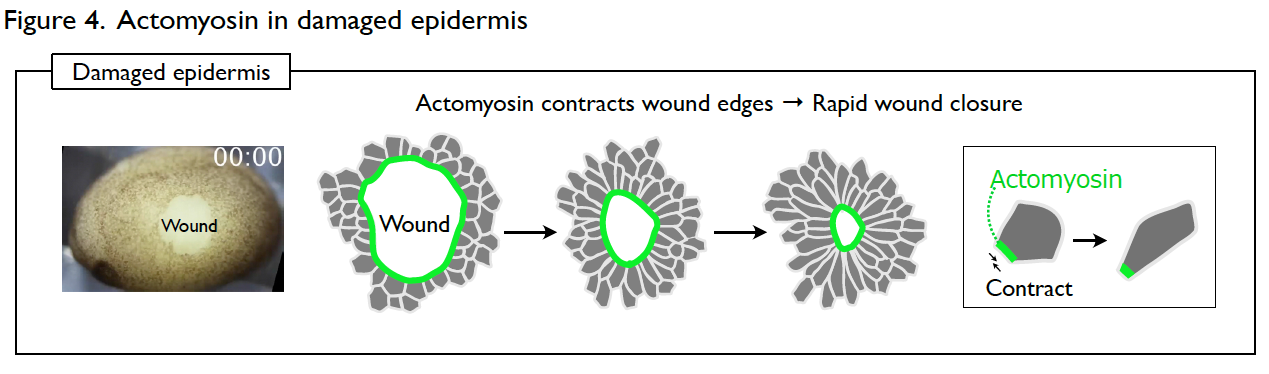
Understanding the molecular regulations of actomyosin is key to addressing this question. Actomyosin is a cytoskeletal element that generates contractile forces in a cell. In the normal and intact epidermis, actomyosin generates contractile forces but is well balanced, leading to a stable cell shape (Fig. 3). However, upon wounding, actomyosin intensively accumulates at the wound edge immediately and contracts the cell edge to close the wound (Fig. 4). The response time of actomyosin to the wounding is surprisingly only three minutes.
We intend to identify the molecular regulations of the actomyosin behavior both in the stable and wounded epidermis. This study will help us understand how the developing epithelial tissues ensure morphological homeostasis.