
Akinobu Onitake, Kunitoshi Yamanaka, Masatoshi Esaki, Teru Ogura.Caenorhabditil elegans fidgetin homolog FIGL-1, a nuclear-localized AAA ATPase, binds to SUMO, Journal of Structural Biology, in press
Fidgetin is a member of the AAA (ATPases associated with diverse cellular activities) chaperones. It is well-known that the specific function of a given AAA protein primarily depends upon its subcellular localization and interacting partners. FIGL-1, a Caenorhabditis elegans homolog of mammalian fidgetin, is localized in the nucleus. Here, we identified that the N-terminal PKRVK sequence of FIGL-1 functions as a monopartite nuclear localization signal. Nuclear localization of FIGL-1 is required for its function. We also found that FIGL-1 specifically interacted with SMO-1, a C. eleganshomolog of small ubiquitin-like modifier (SUMO), using a yeast two-hybrid assay. Furthermore, the direct physical interaction between FIGL-1 and SMO-1 was demonstrated by pull-down assay using purified proteins as well as immunoprecipitation assay using lysates from epitope-tagged SMO-1-expressing worms. Binding of FIGL-1 to SMO-1 is required for its function. The depletion of FIGL-1 and SMO-1 resulted in developmental defects in C. elegans . Taken altogether, our results indicate that FIGL-1 is a nuclear protein and that in concert with SMO-1, FIGL-1 plays an important role in the regulation of C. elegans development.
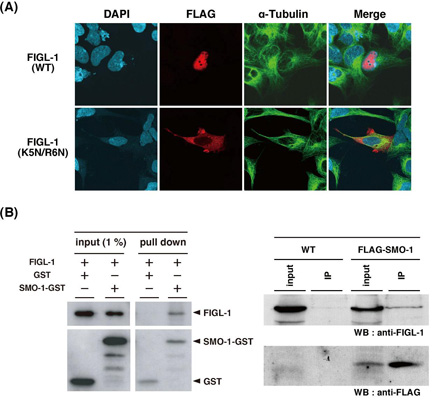
Figure: Nuclear localization and SMO-1 binding of FIGL-1
(A) When FLAG-tagged FIGL-1 is expressed in cultured human HEK293 cells, it is localized in the nucleus. In contrast, when mutated FIGL-1 protein (K5N/R6N) was expressed, this mutant is localized in the cytoplasm. These results suggest that the N-terminal sequence of FIGL-1 is necessary for its nuclear localization.
(B) Purified FIGL-1 and either SMO-1-GST or GST were used for the pull-down assay. FIGL-1 was co-precipitated when it was incubated with SMO-1-GST, but not with GST (Left). Endogenous FIGL-1 was co-precipitated specifically with FLAG-SMO-1 when lysates from FLAG-tagged SMO-1-expressing worms, but not lysates from wild-type worms, were used (Right). Taken together, these results clearly indicate that FIGL-1 interacts with SMO-1 in vitro and in vivo .