
Rie Sawamura, Teru Ogura and Masatoshi Esaki (2014) A conserved α helix of Bcs1, a mitochondrial AAA chaperone, is required for the Respiratory Complex III maturation. Biochem. Biophys. Res. Commun.443: 997-1002.
Mitochondria are important organelle to produce energy for cell activity. Bcs1 (ubiquinol-cytochrome c reductase (bc1) Synthesis) is present in the mitochondrial inner membrane and is required for formation of the respiratory chain complex. Bcs1 is categorized into the AAA family, general functions of which are unfolding and degradation of proteins, but is required for assembly of a protein complex. The human homolog of Bcs1 has been identified as a responsible gene for certain fatal disorders. Thus, elucidation of the functional mechanism of Bcs1 is valuable for understanding these human disorders. It has been reported that Bcs1 is essential for the Complex III assembly, especially for insertion of Rip1 (Rieske iron sulfurprotein) into the premature Complex III in the yeast Saccharomyces cerevisiae. Here, we describe the importance of the N-terminal region of Bcs1 located in the intermembrane space for its function.
The N-terminal region of Bcs1, located in the mitochondrial intermembrane space, is significantly variable among species. We found that an α-helix containing 38th–40th amino acid residues of Bcs1 was crucial for the assembly of Rip1 into the Complex III in Saccharomyces cerevisiae. These residues and the α-helix are conserved in other species, suggesting that only the residues required for function of Bcs1 are selected during evolution.
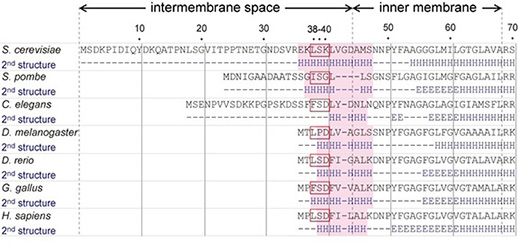
Figure. The N-terminal short segment (red boxes) and α-helix (shaded in pink) of Bcs1 in the intermembrane space, which are highly conserved in eukaryotes, are required for function of Bcs1, H: α-helices, E: β-sheets.